Overview Vaccine Safety
Introduction
Vaccination is one of the most important public health intervention in history. Vaccines prevent more than 2.5 million child deaths each year.
Vaccines used in national immunisation programmes have been rigorously tested for quality, safety and efficacy. However, as with any pharmaceutical product, adverse events may occasionally result from vaccination. The general public has low tolerance to adverse events as vaccines are usually given to healthy persons. Therefore, the expectations towards safety standards is much higher for vaccines as compared to medicines that are given to sick people (Table 1).
An adverse event can be caused or triggered by a vaccine or the process of immunisation. However, many of the symptoms and signs of an adverse event following immunisation (AEFI), can also occur in the absence of immunisation, hence the need to know the extent of the background occurrence. It is therefore important to differentiate between an adverse reaction following immunisation and a co-incidental event.
Vaccine pharmacovigilance is the science and activities relating to the detection, assessment, understanding and communication of adverse events following immunisation and other vaccine- or immunisation-related issues, and to the prevention of untoward effects of the vaccine or immunisation.
Table 1. Differences between vaccine and medicine pharmacovigilance
| Vaccines | Other medicines |
| Prevention in healthy, larger population – Less tolerant to risk |
Treatment in ill, smaller population – More tolerant to risk |
| Limited number of products | Large number of products |
| With single dose, greater potential for temporal “coincidence” | Treatment over time – less “coincidence” after a single dose |
| Cold chain critical | Storage and handling less critical |
| Biological product – more prone to lot variation and instability | Chemical product |
| Mass campaigns – delivery of many doses in a short time to a defined population | No mass campaigns – private prescribing to a less defined population |
| Collaboration between public health/NIP, NRA and manufacturers | Less collaboration between health system/govt/NRA and manufacturers |
Adapted from Prof M. Gold, University of Adelaide
Vaccine quality, safety and efficacy
Vaccines are biological products and it is essential that quality is ensured from the first step in the production process to the final packing of the vaccine. Quality control requires strict compliance with good manufacturing practices (GMP) and with good laboratory practices (GLP).
Safety – the vaccine should be well tolerated and less reactogenic than the disease(s) it targets; should not revert to virulence or be transmitted to others (for live vaccines); and should cause no significant disease.
Quality – the vaccine should be developed and tested according to state-of-the-art science and technology and manufactured under Good Manufacturing Practices in dedicated GMP production facilities.
Efficacy – the vaccine should be highly immunogenic, efficacious and protect against the disease it is targeting.
National Regulatory Authorities (NRAs) ensure the quality, safety and effectiveness of vaccines. Before being introduced into national immunisation programmes, vaccines are assessed for their safety and efficacy in clinical trials. Once introduced, vaccines undergo thorough and continuous reviews of their manufacturing process and NRAs monitor and investigate adverse events to ensure safety for the population.
Pre-licensure assessment of vaccine safety will detect common adverse reaction, but will not detect adverse reactions which are rare, delayed or unexpected, or occur in sub-populations (Table 2). Therefore, post-licensure surveillance is of utmost importance to detect these less common adverse events. The primary mechanism of post-licensure surveillance is passive (spontaneous) reporting by healthcare providers, vaccine recipients and manufacturers.
Table 2. Pre-licensure assessment of vaccine safety
| Trials | Sample size | Adverse reactions | |
| Common | Rare | ||
| Animal trials | +/- | – | |
| Clinical trials | |||
| Phase I | 10-100 | +/- | – |
| Phase II | 100-1.000 | + | – |
| Phase III | 1.000-10.000 | + | – |
Adapted from Prof M. Gold, University of Adelaide
Adverse Events Following Immunisation: definition and classification
An adverse events following immunisation (AEFI) is any untoward medical occurrence (unfavourable or unintended sign, abnormal laboratory finding, symptom or disease) which follows immunisation and which does not necessarily have a causal relationship with the usage of the vaccine. Table 3 provides information on the five major categories of AEFIs as defined by the Council for International Organisations of Medical Sciences (CIOMS) and WHO.
Table 3. Types, description and examples of Adverse Events Following Immunisation
| Type of AEFI | Description | Example |
| Vaccine product- related reaction | AEFI caused or precipitated by a vaccine due to one or more of the properties of the vaccine product | Extensive limb swelling following DTP vaccination |
| Vaccine quality defect-related reaction | AEFI caused or precipitated by a vaccine that is due to one or more quality defects of the product, including its administration device | Failure by manufacturer to completely inactivate a lot of inactivated vaccine |
| Immunisation error-related reaction | AEFI that is caused by inappropriate vaccine handling, prescribing, or administration | Transmission of infection by contaminated multi-dose vial |
| Immunisation anxiety-related reaction | AEFI arising from anxiety about the immunisation | Vasovagal syncope in an adolescent following vaccination |
| Coincidental event | AEFI caused by something other than the vaccine product, immunisation error, or immunisation anxiety | A fever after vaccination (temporal association) and malarial parasite isolated from blood |
Vaccine reactions can be classified by a cause-specific vaccine reaction and by seriousness and frequency (very common, common, uncommon, rare and very rare). Most vaccine reactions are minor and of short duration. Most common local reactions include pain, swelling and redness at injection site. Systemic reactions include fever, irritability and malaise. Serious reactions are very rare.
A serious adverse event can:
- require inpatient hospitalisation
- result in persistent or significant disability
- be life-threatening
- result in congenital anomaly
- result in death
It is important to conduct a causality assessment so that an adverse vaccine or immunisation reaction can be differentiated from a co-incidental event. Some signs, symptoms or diseases may have a “background rate” and occur without immunisation.
Vaccine safety and public trust
In relation to vaccine safety and public trust, many public health experts consider vaccines to be “the victims of their own success”. The higher the vaccination coverage against a disease, the less visible that disease becomes to the general public. As the threat of the disease itself diminishes, public attention is directed to the potential threat of adverse reactions to the vaccine. A distorted perception of the risk of the vaccine, and of the consequences of the prevented illness, can subsequently lead to decreased demand for the vaccine and growing vaccine hesitancy. This can result in delayed, selective, or refused vaccination. This might lead to an outbreak of the disease in the unvaccinated population, which subsequently can restore confidence in the vaccine, as illustrated in Figure 1.
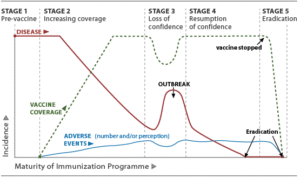
Figure 1. Relation between vaccination coverage and vaccine confidence. Adapted from WHO Vaccine Safety Basics.
Importance of post-licensure surveillance
- Detect, correct and prevent immunisation error-related reactions
- Identify potential problems with vaccine lots or brands
- Ensure that coincidental events do not negatively affect the immunisation programme
- Generate new hypotheses about vaccine reactions that are specific to the population
- Estimate rates of occurrence on AEFI in the local population, compared with trials and international data
- To maintain the confidence of the community and health staff in the immunization programme by quickly responding to concerns about immunization safety
- To effectively communicate with parents, community, the media and other stakeholders to create awareness of AEFIs without jeopardizing the immunization
Further reading
- Tozzi AE, Asturias EJ, Balakrishnan MR, Halsey NA, Law B and Zuber PLF. Assessment of causality of individual adverse events following immunization (AEFI): A WHO tool for global use. Vaccine 2013; 31(44), 5041-5046.
- World Health Organization. Vaccine Safety Basics Learning Manual. Geneva: World Health Organization;
- World Health Organization. Mid-Level Management Course for EPI Managers. Module 9: Immunisation safety. Geneva: World Health Organization;
